
Hill Lab @JohnsHopkins
Summary
The overarching goal of our research is to determine the underlying rules governing the complex dynamics of infectious diseases. We develop mathematical and computational models for this task, and work in close collaboration with experimental labs and clinical researchers. Quantitative models are a powerful tool to test hypotheses about the biological mechanisms responsible for the observed trends in data, and, to integrate existing biological data in a formal way to predict the outcomes of experiments that have not yet, or could never, be performed. Our work bridges a range of data sources, including pathogen genetic data, in vitro microbial growth, time-series characterization of infections and immunity within infected individuals, in vivo lineage tracing, pharmacokinetics and pharmacodynamics, and human behavior.
Click on any of the research themes below to learn more
Since HIV/AIDS emerged around the world nearly forty years ago, it has become one of the deadliest pandemics ever. Incredible progress has been made in understanding and treating the infection, but we still don’t have a cure and haven’t controlled the epidemic. We develop analytic methods, in close collaboration with experimentalists to
- understand the basic biology of the virus, including how it is transmitted, how it evades the immune system, how to maintains latency, and how it causes AIDS
- understand why antiretroviral therapy sometimes fails, for example due to the evolution of drug resistance, and help design optimal treatment regimens
- evaluate the potential of new investigation HIV therapies, such as latency-reversing agents, immunotherapy, and gene therapy
- use HIV as a model system to understand basic evolutionary dynamics
Representative papers:
Rosenbloom DIS*, Hill AL*, Rabi SA*, Siliciano RF, Nowak MA (2012). Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nature Medicine, 18 (9) , 1378-1385. (PubMed)
Hill AL*, Rosenbloom DIS*, Fu F, Nowak MA, Siliciano RF (2014). Predicting the outcomes of treatments to eradicate the latent reservoir for HIV-1. PNAS, 111 (37), 13475-13480. (Arxiv, PubMed)
Hill AL, Rosenbloom DIS, Nowak MA, Siliciano RF (2018). Insight into treatment of HIV infection from viral dynamics models. Immunological Reviews. Sep 1;285(1):9–25.
Related papers:
Simonetti FR, Zhang H, Soroosh G, Duan J, Rhodehouse K, Hill AL, Beg SA, McCormick K, Raymond H, Nobles CL, Everett J, Kwon K, White J, Lai J, Hoh R, Deeks SG, Bushman FD, Siliciano JD, Siliciano RF (2020). Antigen-driven clonal selection shapes the persistence of HIV-1 infected CD4+ T cells Journal of Clinical Investigation. 131. doi.org/10.1172/JCI145254 (PubMed)
Gupta RK, Peppa D, Hill AL, Gálvez C, Salgado M, Pace M, et al (2020). Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. The Lancet HIV. (PubMed)
Whitney JB, Lim S-Y, Osuna CE, Kublin JL, Chen E, Gyeol Y, Liu P-T, Abbink P, Borducchi EN, Hill AL, Lewis MG, Geleziunas R, Robb ML, Michael NL, Barouch DH (2018). Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nature Communications. 9(1):5429 (PubMed)
Hill AL (2018). Modeling HIV persistence and cure studies. Current Opinion in HIV and AIDS, 13 (5), 428-434 (PubMed)
Lim S-Y, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB (2018). TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Science Translational Medicine. May 2;10(439):eaao4521 (PubMed).
Wang Z, Gurule EE, Brennan TP, Gerold JM, Kwon KJ, Hosmane NN, Kumar M, Beg S, Capoferria AA, Ray SC, Ho YC, Hill AL, Siliciano JD, Siliciano RF (2018). Expanded clones carrying replication-competent HIV-1 persist, wax, and wane. PNAS. February: 201720665. doi:10.1073/pnas.1720665115 (PubMed)
Hill AL (2018). Mathematical Models of HIV Latency. In: HIV Latency. Current Topics in Microbiology and Immunology. Springer. (PubMed)
Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Mo SS, Craig M, Mazdiyasni H, Cleveland C, Rogner J, Lee YAL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G (2017). Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nature Communications, 9: 2. doi:10.1038/s41467-017-02294-6.
Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF (2017). Re-evaluating evolution in the HIV reservoir. Nature, Brief Communication Arising, 2017;551(7681):E6 (PubMed, Biorxiv)
Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, NL, Barouch DH (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkey. Nature, 540 (7632), 284-287: doi:10.1038/nature20583 (PubMed)
Hill AL, Rosenbloom DIS, Siliciano JD, Siliciano RF (2016). Insufficient evidence for rare activation of latent HIV in the absence of reservoir-reducing interventions. PLoS Pathogens. Aug 25;12(8):e1005679. doi: 10.1371/journal.ppat.1005679. (PubMed)
Hill AL, Rosenbloom DIS, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF, Henrich TJ (2016) Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathogens, 12(4):e1005535. doi:10.1371/journal.ppat.1005535. ( BioRxiv, PubMed )
Rosenbloom DIS, Elliott O, Hill AL, Henrich TJ, Siliciano JM, Siliciano RF (2015). Designing and interpreting limiting dilution assays: general principles and applications to the latent reservoir for HIV-1. Open Forum InfectiousDiseases, 2(4):ofv123. doi:10.1093/ofid/ofv123. (BioRxiv, PubMed )
Laird GM, Bullen CK, Rosenbloom DIS, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF (2015). Ex vivo analysis identifies effective HIV-1 latency–reversing drug combinations. Journal of Clinical Investigation. doi:10.1172/JCI80142 (PubMed)
Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DIS, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH (2014). Rapid establishment of the viral reservoir prior to systemic viremia following mucosal SIV infection of rhesus monkeys. Nature. 512 (7512): 74-77 (PubMed)
Henrich TJ, Hanhauser E, Marty MF, Sirignano MN, Keating S, Lee T-H, Robles YP, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR (2014). Antiretroviral-free HIV-1 remission and viral rebound following allogeneic stem-cell transplantation: implications for HIV-1 cure research. Annals of Internal Medicine, 161(5):319-327 (PubMed)
Hill AL, Rosenbloom DIS, Nowak MA (2012). Evolutionary dynamics of HIV at multiple spatial and temporal scales. J Mol Med, 90 (5), 543-561. (PubMed)
Early in the COVID-19 pandemic, we developed an model for disease transmission and clinical progression, and deployed it as an easy-to-use web application via R Shiny. This app has been used and adapted by dozens of researchers and agencies around the world, and visited tens of thousands of times. We also provided individualized modeling assistance to many of these groups.
In addition, we are using models to answer other key questions for the epidemic, such as the role of household transmission and population structure in disease dynamics and the efficacy of control measures, the effect of relaxation measures, and the impact of mass evictions on disease spread. We are also working on models connecting within-host disease kinetics and population-level transmission dynamics.
Along with other researchers from the Johns Hopkins Infectious Disease Dynamics Group, we contribute to the COVID-19 Scenario Pipeline, a custom-built software package for mechanistic mathematical modeling, data fitting, and parameter inference for COVID-19 transmission. Projections from this model are regularly contributed to the COVID-19 Scenario Modeling Hub and the COVID-19 Forecast Hub.
In collaboration with researchers at Johns Hopkins International Vaccine Access Center, we led a large systematic review of the efficacy of COVID-19 vaccines used around the world.
Representative papers/resources:
Modeling COVID-19 Spread vs Healthcare Capacity: An R Shiny App
Nande A, Adlam B, Sheen J, Levy MZ, Hill AL (2020). Dynamics of COVID-19 under social distancing measures are driven by transmission network structure. PLOS Computational Biology. 17 e1008684 doi.org/10.1371/journal.pcbi.1008684 (medRxiv, PubMed)
Nande A, Sheen J, Walters EL, Klein B, Chinazzi M, Gheorghe A, Adlam B, Shinnick J, Tejeda MF, Scarpino SV, Vespignani A, Greenlee AJ, Schneider D, Levy MZ, Hill AL (2021). The effect of eviction moratoria on the transmission of SARS-CoV-2. Nature Communications. (medRxiv, PubMed)
Related papers:
Higdon MM, Wahl B, Jones CB, Rosen JG, Truelove SA, Baidya A, Nande AA, ZhamaeiZadeh PA, Walter KK, Feikin DR, Patel MK, Knoll MD, Hill AL (2022). A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. Open Forum Infectious Diseases. 9: ofac138. doi:10.1093/ofid/ofac13 (medRxiv)
Truelove S, Smith CP, Qin M, Mullany LC, Borchering RK, Lessler J, et al .. Hill AL et al.… Runge MC, Viboud C (2022). Projected resurgence of COVID-19 in the United States in July—December 2021 resulting from the increased transmissibility of the Delta variant and faltering vaccination. eLife. 11: e73584. doi:10.7554/eLife.73584 (medRxiv, PubMed)
Cramer EY, Ray EL, Lopez VK, Bracher J, et al. …Hill AL, et al. .. Reich NG (2022). Evaluation of individual and ensemble probabilistic forecasts of COVID-19 mortality in the United States. PNAS. 119 (15), e2113561119. doi:10.1073/pnas.2113561119 (medRxiv, PubMed)
Rader B, Scarpino S, Nande A, Hill AL, Adlam B, Reiner RC, Pigott DM, Gutierrez B, Zarebski A, Shrestha M, Brownstein JS, Castro MC, Dye C, Tian Y, Pybus OG, Kraemer MUG (2020). Crowding and the epidemic intensity of COVID-19 transmission. Nature Medicine. 26, 1829–1834 (medRxiv, PubMed)
Many microbial infections undergo continuous, rapid evolution in response to host defenses and medical interventions, making them difficult to prevent or treat. We use evolutionary theory to understand the factors that influence infectious disease evolution both within and between individuals. In particular, we examine realistic aspects of the environment pathogens are exposed to - such as temporal and spatial heterogeneity in selection pressures, and co-evolution with host defenses. We aim to explain observed patterns of genotypic and phenotypic diversity in pathogens, but also to hypothesize about yet-unobserved trends.
Our goals of this work are two-fold. Firstly, we hope to help design better interventions and reduce the public health burden of infectious disease. Secondly, we aim to exploit the rapid and repeatable nature of pathogen adaptation to inform the more general study of evolutionary dynamics.
Representative papers:
Krieger MS, Denison CE, Anderson TL, Nowak MA, Hill AL (2020). Population structure across scales facilitates coexistence and spatial heterogeneity of antibiotic-resistant infections. PLOS Computational Biology. 2020;16: e1008010
Leventhal GE*, Hill AL*, Nowak MA, Bonhoeffer S (2015). Evolution and emergence of infectious diseases in theoretical and real world networks. Nature Communications, 6 (6101); doi:10.1038/ncomms7101 (PubMed)
Related papers:
Neagu IA, Olejarz J, Freeman M, Rosenbloom DIS, Nowak MA, Hill AL (2018). Life cycle synchronization is a viral drug resistance mechanism. PLoS Computational Biology. 14: e1005947. doi:10.1371/journal.pcbi.1005947 (PubMed)
Moreno-Gámez S*, Hill AL*, Rosenbloom DIR, Petrov D, Nowak MA, Pennings P (2015). Imperfect drug penetration leads to spatial monotherapy and rapid evolution of multi-drug resistance. PNAS, 112(22):E2874-E2883. doi:10.1073/pnas.1424184112. (BioRxiv, PubMed)
Humplik J, Hill AL, Nowak MA (2014). Evolution of infectious diseases in finite populations. Journal of Theoretical Biology, 360 (149-162) (PDF, PubMed)
Rosenbloom DIS*, Hill AL*, Rabi SA*, Siliciano RF, Nowak MA (2012). Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nature Medicine, 18 (9) , 1378-1385. (PubMed)
Hill AL, Rosenbloom DIS, Nowak MA (2012). Evolutionary dynamics of HIV at multiple spatial and temporal scales. J Mol Med, 90 (5), 543-561. (PubMed)
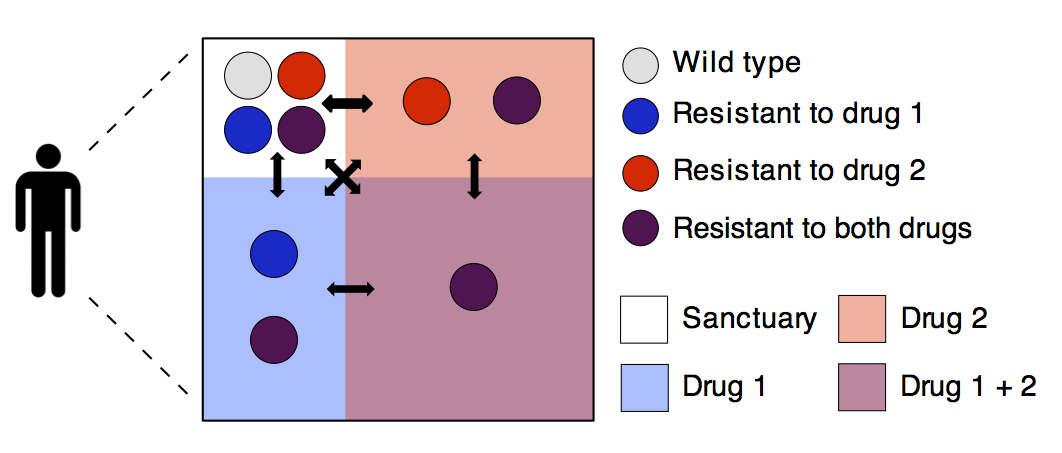
Drugs that specifically target pathogens have been one of the major medical breakthroughs of the last century, but their success is continually under threat due to the evolution of drug resistant strains. We use evolutionary theory combined with data from a range of sources to determine factors that increase or decrease the risk of drug resistance, to evaluate the predictability of resistance evolution, or to suggest new lab experiments or clinical trials of drugs.
Representative papers:
Nande A , Hill AL (2022). The risk of drug resistance during long-acting antimicrobial therapy. In review (medRxiv)
Krieger MS, Denison CE, Anderson TL, Nowak MA, Hill AL (2020). Population structure across scales facilitates coexistence and spatial heterogeneity of antibiotic-resistant infections. PLOS Computational Biology. 2020;16: e1008010
Moreno-Gámez S*, Hill AL*, Rosenbloom DIR, Petrov D, Nowak MA, Pennings P (2015). Imperfect drug penetration leads to spatial monotherapy and rapid evolution of multi-drug resistance. PNAS, 112(22):E2874-E2883. doi:10.1073/pnas.1424184112. (BioRxiv, PubMed)
Neagu IA, Olejarz J, Freeman M, Rosenbloom DIS, Nowak MA, Hill AL (2018). Life cycle synchronization is a viral drug resistance mechanism. PLoS Computational Biology. 14: e1005947. doi:10.1371/journal.pcbi.1005947 (PubMed)
Related papers:
Hill AL, Rosenbloom DIS, Nowak MA, Siliciano RF (2018). Insight into treatment of HIV infection from viral dynamics models. Immunological Reviews. Sep 1;285(1):9–25. +details
Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Mo SS, Craig M, Mazdiyasni H, Cleveland C, Rogner J, Lee YAL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G (2017). Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nature Communications, 9: 2. doi:10.1038/s41467-017-02294-6.
Rosenbloom DIS*, Hill AL*, Rabi SA*, Siliciano RF, Nowak MA (2012). Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nature Medicine, 18 (9) , 1378-1385. (PubMed)
Hill AL, Rosenbloom DIS, Nowak MA (2012). Evolutionary dynamics of HIV at multiple spatial and temporal scales. J Mol Med, 90 (5), 543-561. (PubMed)
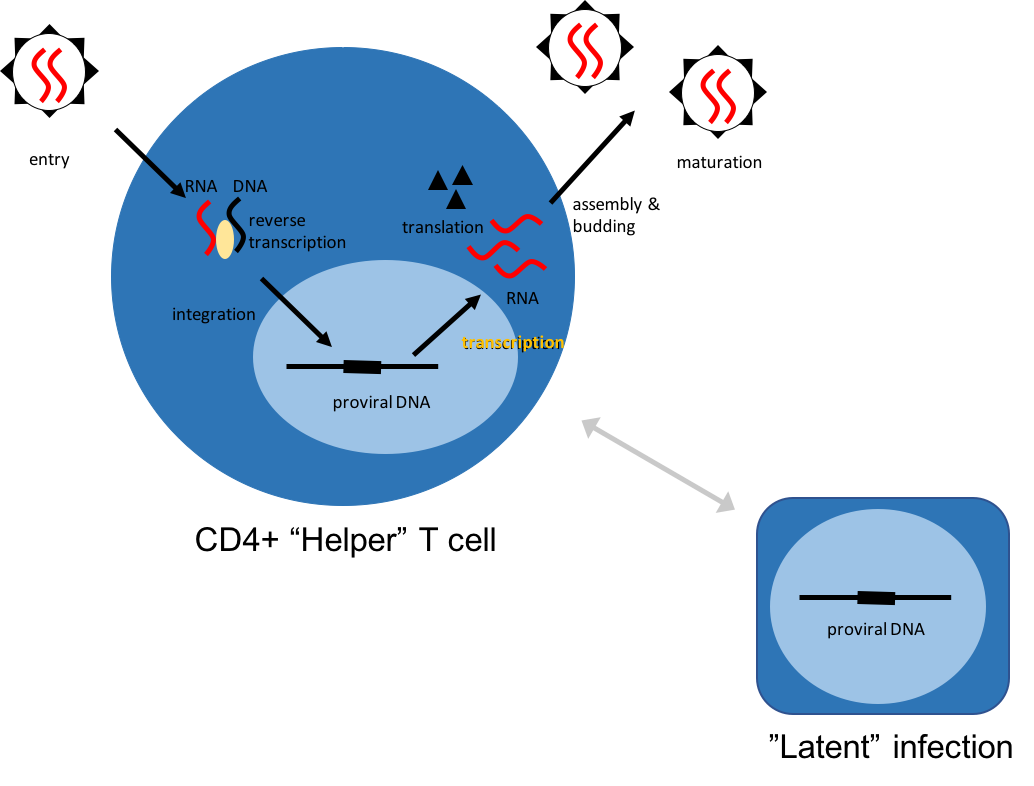
Many different viral, bacterial, and parasitic infections can enter “latent” phases or maintain chronic infections by other means. This is in contrast to most infections which can be rapidly cleared by the immune system or antimicrobial therapy. We are broadly interested in the mechanisms by which infections achieve long-term persistence. This is particularly important for HIV, since latency is one of the main reasons we still cannot cure the infection. It is also critical for cancer-causing viruses, like the Kaposi’s sarcoma virus. We develop methods to decipher the cellular and viral processes maintaining latency, and build models to evaluate the potential of different strategies to perturb latency and clear infections.
Representative paper:
Hill AL*, Rosenbloom DIS*, Fu F, Nowak MA, Siliciano RF (2014). Predicting the outcomes of treatments to eradicate the latent reservoir for HIV-1. PNAS, 111 (37), 13475-13480. (Arxiv, PubMed)
Hill AL (2018). Mathematical Models of HIV Latency. In: HIV Latency. Current Topics in Microbiology and Immunology. Springer (PubMed)
Related papers:
Simonetti FR, Zhang H, Soroosh G, Duan J, Rhodehouse K, Hill AL, Beg SA, McCormick K, Raymond H, Nobles CL, Everett J, Kwon K, White J, Lai J, Hoh R, Deeks SG, Bushman FD, Siliciano JD, Siliciano RF (2020). Antigen-driven clonal selection shapes the persistence of HIV-1 infected CD4+ T cells Journal of Clinical Investigation. 131. doi.org/10.1172/JCI145254 (PubMed)
Gupta RK, Peppa D, Hill AL, Gálvez C, Salgado M, Pace M, et al (2020). Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. The Lancet HIV. (PubMed)
Whitney JB, Lim S-Y, Osuna CE, Kublin JL, Chen E, Gyeol Y, Liu P-T, Abbink P, Borducchi EN, Hill AL, Lewis MG, Geleziunas R, Robb ML, Michael NL, Barouch DH (2018). Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nature Communications. 9(1):5429 (PubMed)
Hill AL, Rosenbloom DIS, Nowak MA, Siliciano RF (2018). Insight into treatment of HIV infection from viral dynamics models. Immunological Reviews. Sep 1;285(1):9–25.
Hill AL (2018). Modeling HIV persistence and cure studies. Current Opinion in HIV and AIDS, 13 (5), 428-434 (PubMed)
Lim S-Y, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB (2018). TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Science Translational Medicine. May 2;10(439):eaao4521 (PubMed).
Wang Z, Gurule EE, Brennan TP, Gerold JM, Kwon KJ, Hosmane NN, Kumar M, Beg S, Capoferria AA, Ray SC, Ho YC, Hill AL, Siliciano JD, Siliciano RF (2018). Expanded clones carrying replication-competent HIV-1 persist, wax, and wane. PNAS. February: 201720665. doi:10.1073/pnas.1720665115 (PubMed)
Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF (2017). Re-evaluating evolution in the HIV reservoir. Nature, Brief Communication Arising, 2017;551(7681):E6 (PubMed, Biorxiv)
Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, NL, Barouch DH (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkey. Nature, 540 (7632), 284-287: doi:10.1038/nature20583 (PubMed)
Hill AL , Rosenbloom DIS, Siliciano JD, Siliciano RF (2016). Insufficient evidence for rare activation of latent HIV in the absence of reservoir-reducing interventions. PLoS Pathogens. Aug 25;12(8):e1005679. doi: 10.1371/journal.ppat.1005679. (PubMed)
Hill AL, Rosenbloom DIS, Goldstein E, Hanhauser E, Kuritzkes DR, Siliciano RF, Henrich TJ (2016) Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathogens, 12(4):e1005535. doi:10.1371/journal.ppat.1005535. ( BioRxiv, PubMed )
Rosenbloom DIS, Elliott O, Hill AL, Henrich TJ, Siliciano JM, Siliciano RF (2015). Designing and interpreting limiting dilution assays: general principles and applications to the latent reservoir for HIV-1. Open Forum InfectiousDiseases, 2(4):ofv123. doi:10.1093/ofid/ofv123. (BioRxiv, PubMed )
Laird GM, Bullen CK, Rosenbloom DIS, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF (2015). Ex vivo analysis identifies effective HIV-1 latency–reversing drug combinations. Journal of Clinical Investigation. doi:10.1172/JCI80142 (PubMed)
Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DIS, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH (2014). Rapid establishment of the viral reservoir prior to systemic viremia following mucosal SIV infection of rhesus monkeys. Nature. 512 (7512): 74-77 (PubMed)
Henrich TJ, Hanhauser E, Marty MF, Sirignano MN, Keating S, Lee T-H, Robles YP, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR (2014). Antiretroviral-free HIV-1 remission and viral rebound following allogeneic stem-cell transplantation: implications for HIV-1 cure research. Annals of Internal Medicine, 161(5):319-327 (PubMed)

Bed bug infestations have resurged in cities around the world, but remain a neglected public health problem. In 2016 we began participating in an interdiscplinary working group on bed bugs hosted at the NSF-sponsored National Socio-Ecological Synthesis Center. Since then, we have been assisting with the development of mathematical models and analysis of bed bug transmission and the connection with the housing market.
Related papers:
Xie S, Hill AL, Rehmann CR, Levy MZ (2018). Dynamics of bed bug infestations and control under disclosure policies. PNAS. 116 (13), 6473-6481 (PubMed).
Hacker KP, Greenlee AJ, Hill AL, Schneider D, Levy MZ (2022). Spatiotemporal trends in bed bug metrics: New York City. (medRxiv)
How do bed bugs effect landlords and tenants? Science Journal for Kids
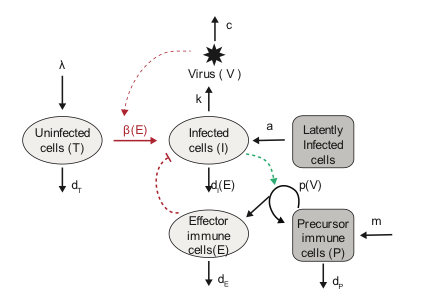
Infectious diseases display complex non-linear dynamics, and we are often interested in knowing what the underlying mechanisms generating these dynamics are or what the value of particular parameters are and how they change in different settings. We develop and employ methods to infer mechanistic dynamic models from a variety of data sources. We are working on Bayesian methods for deterministic and stochastic models, tools to identify treatment effects, unifying predictive techniques of statistical and mathematical models, harnessing ideas from machine learning for dynamical systems, and integrating various data sources in principled ways. We are interested in applying these methods beyond the infection context, including to understand dynamics in the immune system and properties of cell turnover.
Related papers:
Prague M, Gerold JM, Balelli I, Pasin C, Li JZ, Barouch DB, Whitney JB, Hill AL (2020). Viral rebound kinetics following single and combination immunotherapy for HIV/SIV. (Biorxiv)
Lim S-Y, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB (2018). TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Science Translational Medicine. May 2;10(439):eaao4521 (PubMed).
Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, NL, Barouch DH (2016). Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkey. Nature, 540 (7632), 284-287: doi:10.1038/nature20583 (PubMed)
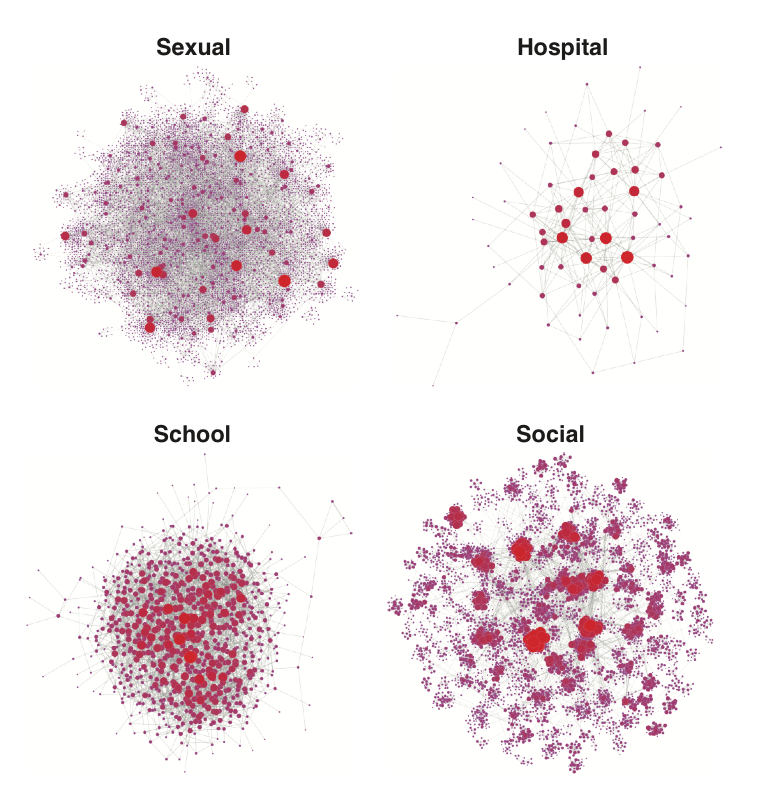
The standard models of infection dynamics assume that all hosts are equal - equally susceptible to infection, able to spread disease to other contacts at equal rates, and equally likely to be treated. In reality, this is rarely the case, and heterogeneities in host populations can lead to very different dynamics and evolution of infections than would arise by just considering an “average” individual. We develop models to understand these effects and methods to infer population structure from data.
Related papers:
Nande A, Adlam B, Sheen J, Levy MZ, Hill AL (2021). Dynamics of COVID-19 under social distancing measures are driven by transmission network structure. PLOS Computational Biology. 17 e1008684 doi.org/10.1371/journal.pcbi.1008684 (medRxiv, PubMed)
Rader B, Scarpino S, Nande A, Hill AL, Adlam B, Reiner RC, Pigott DM, Gutierrez B, Zarebski A, Shrestha M, Brownstein JS, Castro MC, Dye C, Tian Y, Pybus OG, Kraemer MUG (2020). Crowding and the epidemic intensity of COVID-19 transmission. Nature Medicine. 26, 1829–1834 (medRxiv, PubMed)
Nande A, Sheen J, Walters EL, Klein B, Chinazzi M, Gheorghe A, Adlam B, Shinnick J, Tejeda MF, Scarpino SV, Vespignani A, Greenlee AJ, Schneider D, Levy MZ, Hill AL (2021). The effect of eviction moratoria on the transmission of SARS-CoV-2. Nature Communications. (medRxiv, PubMed)
Krieger MS, Denison CE, Anderson TL, Nowak MA, Hill AL (2020). Population structure across scales facilitates coexistence and spatial heterogeneity of antibiotic-resistant infections. PLOS Computational Biology. 2020;16: e1008010
Leventhal GE*, Hill AL*, Nowak MA, Bonhoeffer S (2015). Evolution and emergence of infectious diseases in theoretical and real world networks. Nature Communications, 6 (6101); doi:10.1038/ncomms7101 (PubMed)
Moreno-Gámez S*, Hill AL*, Rosenbloom DIR, Petrov D, Nowak MA, Pennings P (2015). Imperfect drug penetration leads to spatial monotherapy and rapid evolution of multi-drug resistance. PNAS, 112(22):E2874-E2883. doi:10.1073/pnas.1424184112. (BioRxiv, PubMed)
Hill AL, Rand DG, Nowak MA, Christakis NC (2010) Infectious disease modeling of social contagion in networks. PLoS Computational Biology, 6 (11). (PubMed)
Hill AL*, Rand DG*, Nowak MA, Christakis NC (2010) Emotions as infectious diseases in a large social network: the SISa model. Proc Roy Soc B, 277 (1701). (PubMed)
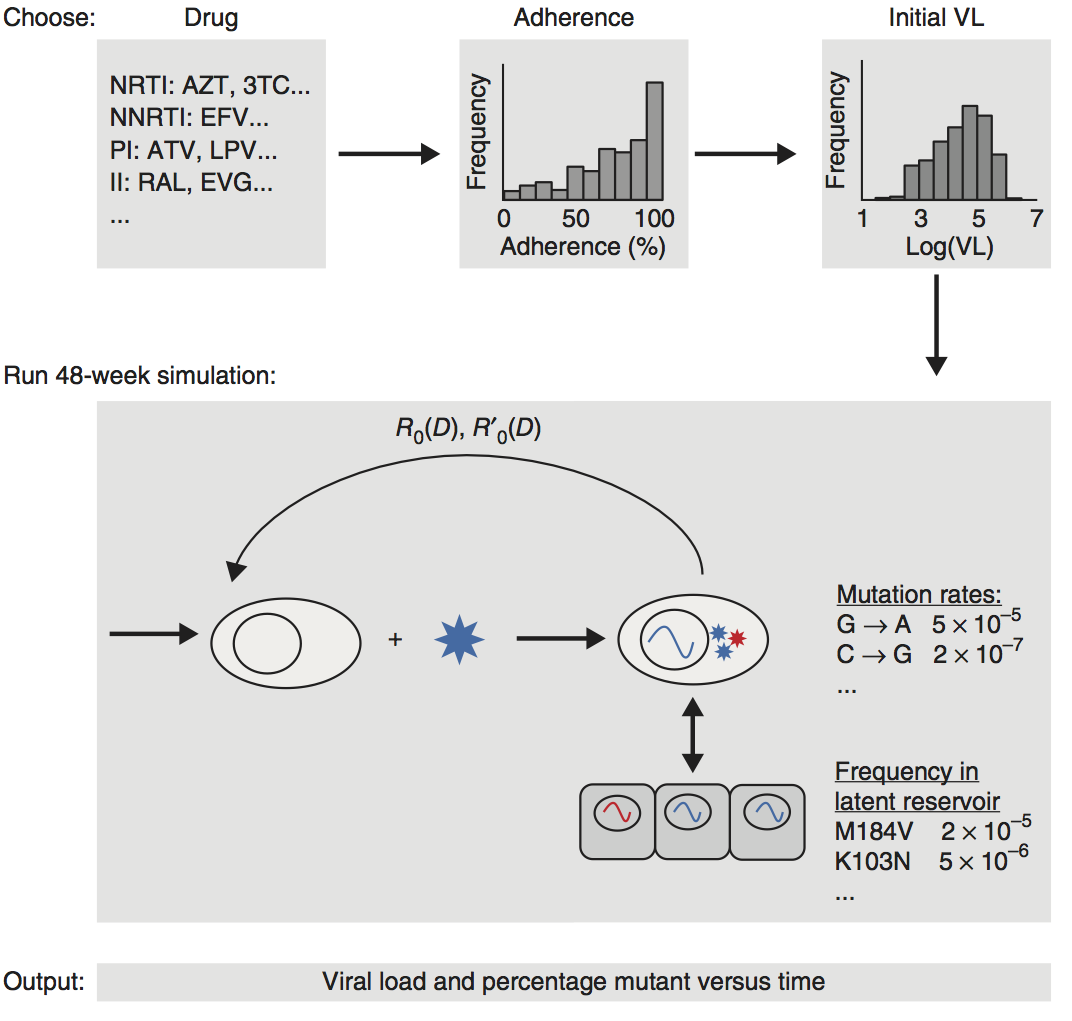
Developing new drugs is extremely expensive and error prone. "Quantitative systems pharmacology” (QSP) is an approach that uses mechanistic mathematical models to describe the interaction between drug molecules and human/animal physiology. It combines the study of pharmacokinetics (the spatial and temporal changes in drug levels in the body due to absorption, metabolism, distribution, and clearance), pharmacodynamics (the drug-concentration-dependent therapeutic effect of the drug), and biomathematical models of disease pathogenesis. QSP can help optimize the drug-development process at all stages. We apply this approach to developing and optimizing therapies for HIV and other infectious diseases.
Related papers:
Nande AA, Hill AL (2022). The risk of drug resistance during long-acting antimicrobial therapy. Submitted (medRxiv)
Hill AL, Rosenbloom DIS, Nowak MA, Siliciano RF (2018). Insight into treatment of HIV infection from viral dynamics models. Immunological Reviews. Sep 1;285(1):9–25. +details
Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Mo SS, Craig M, Mazdiyasni H, Cleveland C, Rogner J, Lee YAL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G (2017). Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nature Communications, 9: 2. doi:10.1038/s41467-017-02294-6.
Rosenbloom DIS*, Hill AL*, Rabi SA*, Siliciano RF, Nowak MA (2012). Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nature Medicine, 18 (9) , 1378-1385. (PubMed)
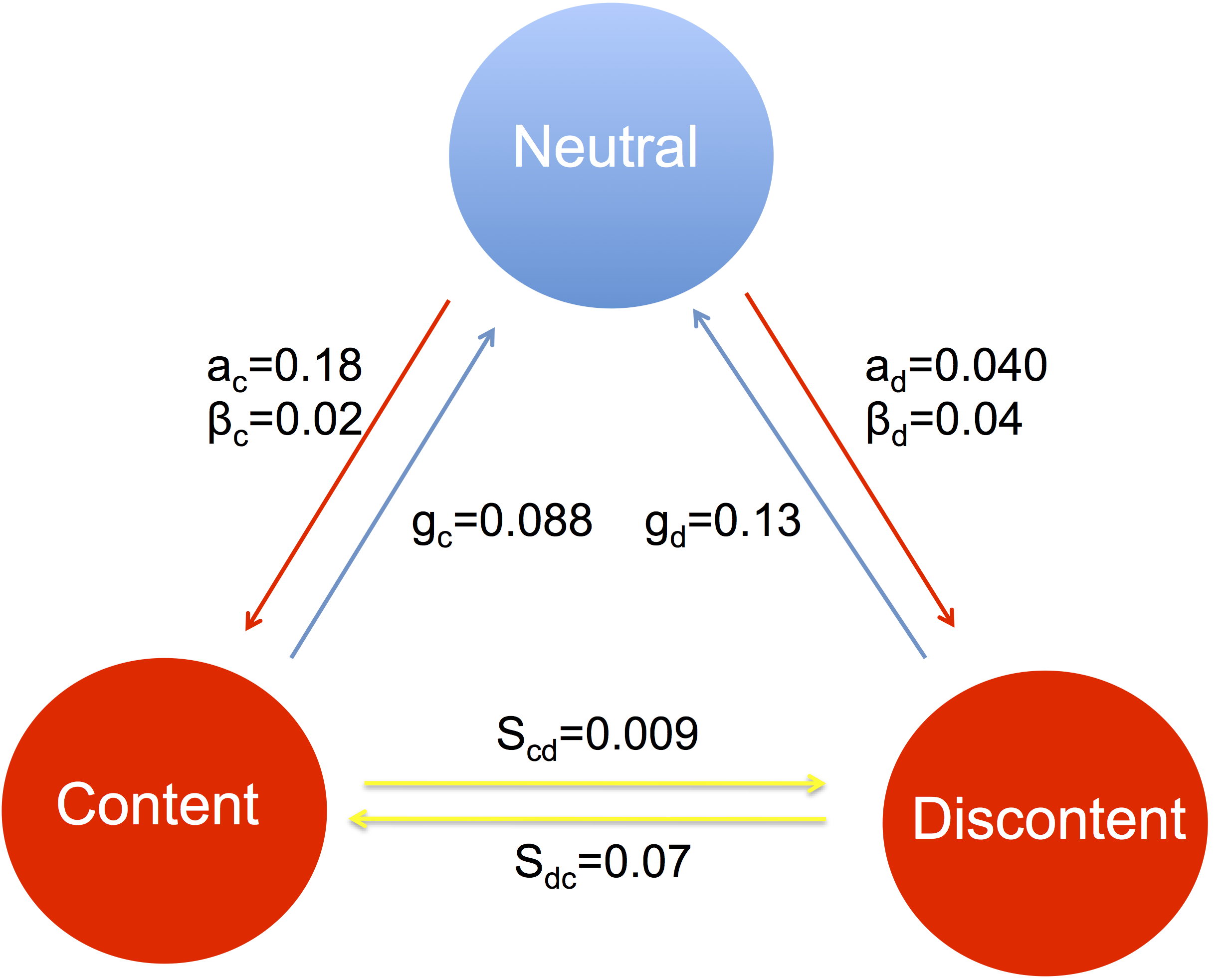
Individuals influence the behavior of their social contacts, and sometimes behaviors, ideas, or even non-communicable health states can “spread” between individuals in ways that resemble the spread of infections. In the past, we have used methods from mathematical epidemiology and network theory to study the effect of social networks on the spread of health-related behaviors in large cohort studies. This led to new ways to quantify the effects of social contagion, to predict future trends, to understand changes in past incidence, and to assess interventions. More generally, we are interested in the inter-personal dynamics of disease risk modifying behavior.
Related papers:
Hill AL, Rand DG, Nowak MA, Christakis NC (2010) Infectious disease modeling of social contagion in networks. PLoS Computational Biology, 6 (11). (PubMed)
Hill AL*, Rand DG*, Nowak MA, Christakis NC (2010) Emotions as infectious diseases in a large social network: the SISa model. Proc Roy Soc B, 277 (1701). (PubMed)